Abstract
Aims/hypothesis
Psychiatric disorders, such as schizophrenia (SCZ), major depressive disorder (MDD) and bipolar disorder (BPD), are highly comorbid with type 2 diabetes. However, the mechanisms underlying such comorbidity are understudied. This study explored the familial aggregation of common psychiatric disorders and type 2 diabetes by testing family history association, and investigated the shared genetic loading between them by testing the polygenic risk score (PRS) association.
Methods
A total of 105,184 participants were recruited from the Taiwan Biobank, and genome-wide genotyping data were available for 95,238 participants. The Psychiatric Genomics Consortium-derived PRS for SCZ, MDD and BPD was calculated. Logistic regression was used to estimate the OR with CIs between a family history of SCZ/MDD/BPD and a family history of type 2 diabetes, and between the PRS and the risk of type 2 diabetes.
Results
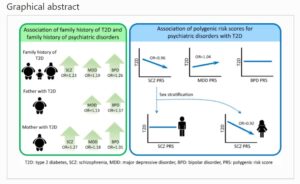
A family history of type 2 diabetes was associated with a family history of SCZ (OR 1.23, 95% CI 1.08, 1.40), MDD (OR 1.19, 95% CI 1.13, 1.26) and BPD (OR 1.26, 95% CI 1.15, 1.39). Compared with paternal type 2 diabetes, maternal type 2 diabetes was associated with a higher risk of a family history of SCZ. SCZ PRS was negatively associated with type 2 diabetes in women (OR 0.92, 95% CI 0.88, 0.97), but not in men; the effect of SCZ PRS reduced after adjusting for BMI. MDD PRS was positively associated with type 2 diabetes (OR 1.04, 95% CI 1.00, 1.07); the effect of MDD PRS reduced after adjusting for BMI or smoking. BPD PRS was not associated with type 2 diabetes.
Conclusions/interpretation
The comorbidity of type 2 diabetes with psychiatric disorders may be explained by shared familial factors. The shared polygenic loading between MDD and type 2 diabetes implies not only pleiotropy but also a shared genetic aetiology for the mechanism behind the comorbidity. The negative correlation between polygenic loading for SCZ and type 2 diabetes implies the role of environmental factors.
Graphical abstract
Data availability
The data that support the findings of this study are available from the Taiwan Biobank. GWAS summary statistics for SCZ, MDD and BPD are available at the PGC website https://www.med.unc.edu/pgc/. GWAS summary statistics for Asian type 2 diabetes are available at https://blog.nus.edu.sg/agen/summary-statistics/t2d-2020/; those for European type 2 diabetes are available at http://diagram-consortium.org/downloads.html
Abbreviations
aOR:
Adjusted odds ratio
BPD:
Bipolar disorder
EAS:
East Asian population
EUR:
European population
GWAS:
Genome-wide association study
LD:
Linkage disequilibrium
MDD:
Major depressive disorder
PC:
Principal component
PGC:
Psychiatric Genomics Consortium
PRS:
Polygenic risk score
SCZ:
Schizophrenia
TWB:
Taiwan Biobank
Nutrigenomics Institute is not responsible for the comments and opinions included in this article
- Mei-Hsin Su,
- Ying-Hsiu Shih,
- Yen-Feng Lin,
- Pei-Chun Chen,
- Chia-Yen Chen,
- Po-Chang Hsiao,
- Yi-Jiun Pan,
- Yu-Li Liu,
- Shih-Jen Tsai,
- Po-Hsiu Kuo,
- Chi-Shin Wu,
- Yen-Tsung Huang &
- Shi-Heng Wang
Diabetologia (2022)Cite this article
- 302 Accesses
- 6 Altmetric
References
- Das-Munshi J, Ashworth M, Dewey ME et al (2017) Type 2 diabetes mellitus in people with severe mental illness: inequalities by ethnicity and age. Cross-sectional analysis of 588 408 records from the UK. Diabet Med 34(7):916–924. https://doi.org/10.1111/dme.13298
- Ward M, Druss B (2015) The epidemiology of diabetes in psychotic disorders. Lancet Psychiatry 2(5):431–451. https://doi.org/10.1016/S2215-0366(15)00007-3
- Calkin C, Gardner D, Ransom T, Alda M (2013) The relationship between bipolar disorder and type 2 diabetes: more than just co-morbid disorders. Ann Med 45(2):171–181
- Lustman P, Clouse R (2005) Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complicat 19(2):113–122
- Wu C, Hsu L, Wang S (2020) Association of depression and diabetes complications and mortality: a population-based cohort study. Epidemiol Psychiatr Sci 29:e96
- Toender A, Vestergaard M, Munk-Olsen T, Larsen J, Kristensen J, Laursen T (2020) Risk of diabetic complications and subsequent mortality among individuals with schizophrenia and diabetes – a population-based register study. Schizophr Res 218:99–106. https://doi.org/10.1016/j.schres.2020.01.024
- van Welie H, Derks EM, Verweij KH, de Valk HW, Kahn RS, Cahn W (2013) The prevalence of diabetes mellitus is increased in relatives of patients with a non-affective psychotic disorder. Schizophr Res 143(2–3):354–357
- Mothi SS, Tandon N, Padmanabhan J et al (2015) Increased cardiometabolic dysfunction in first-degree relatives of patients with psychotic disorders. Schizophr Res 165(1):103–107. https://doi.org/10.1016/j.schres.2015.03.034
- Huang MH, Chen MH, Huang KL et al (2019) Increased risk of type 2 diabetes among the siblings of patients with schizophrenia. CNS Spectrums 24(4):453–459. https://doi.org/10.1017/S1092852918001396
- Kan C, Pedersen NL, Christensen K et al (2016) Genetic overlap between type 2 diabetes and depression in Swedish and Danish twin registries. Mol Psychiatry 21(7):903–909. https://doi.org/10.1038/mp.2016.28
- Winham SJ, Cuellar-Barboza AB, Oliveros A et al (2014) Genome-wide association study of bipolar disorder accounting for effect of body mass index identifies a new risk allele in TCF7L2. Mol Psychiatry 19(9):1010–1016. https://doi.org/10.1038/mp.2013.159
- Haljas K, Amare AT, Alizadeh BZ et al (2018) Bivariate genome-wide association study of depressive symptoms with type 2 diabetes and quantitative glycemic traits. Psychosom Med 80(3):242–251. https://doi.org/10.1097/PSY.0000000000000555
- International Schizophrenia Consortium, Purcell SM, Wray NR et al (2009) Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460(7256):748–752. https://doi.org/10.1038/nature08185
- Clarke TK, Obsteter J, Hall LS et al (2017) Investigating shared aetiology between type 2 diabetes and major depressive disorder in a population based cohort. Am J Med Genet B Neuropsychiatr Genet 174(3):227–234. https://doi.org/10.1002/ajmg.b.32478
- Padmanabhan JL, Nanda P, Tandon N et al (2016) Polygenic risk for type 2 diabetes mellitus among individuals with psychosis and their relatives. J Psychiatr Res 77:52–58. https://doi.org/10.1016/j.jpsychires.2016.02.015
- Foley DL, Mackinnon A, Morgan VA et al (2016) Common familial risk factors for schizophrenia and diabetes mellitus. Aust N Z J Psychiatry 50(5):488–494. https://doi.org/10.1177/0004867415595715
- Fernandez-Egea E, Miller B, Bernardo M, Donner T, Kirkpatrick B (2008) Parental history of type 2 diabetes in patients with nonaffective psychosis. Schizophr Res 98(1–3):302–306
- Chen CH, Yang JH, Chiang CWK et al (2016) Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan biobank project. Hum Mol Genet 25(24):5321–5331. https://doi.org/10.1093/hmg/ddw346
- Fan C-T, Lin J-C, Lee C-H (2008) Taiwan biobank: a project aiming to aid Taiwan’s transition into a biomedical island. Pharmacogenomics 9:235–246. https://doi.org/10.2217/14622416.9.2.235
- 1000 Genomes Project Consortium, Auton A, Brooks L et al (2015) A global reference for human genetic variation. Nature 526(7571):68–74. https://doi.org/10.1038/nature15393
- Lancaster F. Genetic and Quantitative Aspects of Genealogy 2005 [updated 2015. Available from: http://www.genetic-genealogy.co.uk/Toc115570135.html
- Lam M, Chen CY, Li Z et al (2019) Comparative genetic architectures of schizophrenia in east Asian and European populations. Nat Genet 51(12):1670–1678. https://doi.org/10.1038/s41588-019-0512-x
- Howard DM, Adams MJ, Clarke TK et al (2019) Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 22(3):343–352. https://doi.org/10.1038/s41593-018-0326-7
- Giannakopoulou O, Lin K, Meng X et al (2021) The genetic architecture of depression in individuals of east Asian ancestry: a genome-wide association study. JAMA Psychiatry 78(11):1258–1269. https://doi.org/10.1001/jamapsychiatry.2021.2099
- Stahl EA, Breen G, Forstner AJ et al (2019) Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet 51(5):793–803. https://doi.org/10.1038/s41588-019-0397-8
- Spracklen C, Horikoshi M, Kim Y et al (2020) Identification of type 2 diabetes loci in 433,540 east Asian individuals. Nature 582(7811):240–245. https://doi.org/10.1038/s41586-020-2263-3
- Chang C, Chow C, Tellier L, Vattikuti S, Purcell S, Lee J (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4:7
- Bulik-Sullivan B, Finucane HK, Anttila V et al (2015) An atlas of genetic correlations across human diseases and traits. Nat Genet 47(11):1236–1241. https://doi.org/10.1038/ng.3406
- Mahajan A, Taliun D, Thurner M et al (2018) Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet 50(11):1505–1513. https://doi.org/10.1038/s41588-018-0241-6
- Schizophrenia Psychiatric Genome-Wide Association Study Consortium (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511(7510):421–427. https://doi.org/10.1038/nature13595
- Maassen J, ‘T Hart L, Van Essen E et al (2004) Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes Care 53(Suppl 1):S103-S109.
- van den Ouweland J, Lemkes H, Ruitenbeek W et al (1992) Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet 1(5):368–371. https://doi.org/10.1038/ng0892-368
- Machado A, Pan A, da Silva T, Duong A, Andreazza A (2016) Upstream pathways controlling mitochondrial function in major psychosis: a focus on bipolar disorder. Can J Psychiatr 61(8):446–456. https://doi.org/10.1177/0706743716648297
- Lichtenstein P, Yip B, Björk C et al (2009) Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 373(9659):234–239. https://doi.org/10.1016/S0140-6736(09)60072-6
- Perry B, Jones H, Richardson T et al (2020) Common mechanisms for type 2 diabetes and psychosis: findings from a prospective birth cohort. Schizophr Res 223:227–235. https://doi.org/10.1016/j.schres.2020.08.006
- Hagenaars S, Coleman J, Choi S et al (2020) Genetic comorbidity between major depression and cardio-metabolic traits, stratified by age at onset of major depression. Am J Med Genet B Neuropsychiatr Genet 183(6):309–330. https://doi.org/10.1002/ajmg.b.32807
- Cao H, Chen J, Meyer-Lindenberg A, Schwarz E (2017) A polygenic score for schizophrenia predicts glycemic control. Transl Psychiatry 7(12):1295
- Rajkumar AP, Horsdal HT, Wimberley T et al (2017) Endogenous and antipsychotic-related risks for diabetes mellitus in young people with schizophrenia: a Danish population-based cohort study. Am J Psychiatry 174(7):686–694. https://doi.org/10.1176/appi.ajp.2016.16040442
- Vancampfort D, Correll CU, Galling B et al (2016) Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry 15(2):166–174. https://doi.org/10.1002/wps.20309
- Janney C, Fagiolii A, Swartz H, Jakicic J, Holleman R, Richardson C (2014) Are adults with bipolar disorder active? Objectively measured physical activity and sedentary behavior using accelerometry. J Affect Disord 152-154:498–504. https://doi.org/10.1016/j.jad.2013.09.009
- Zheng Y, Ley S, Hu F (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14(2):88–98. https://doi.org/10.1038/nrendo.2017.151
- Pandey A, Chawla S, Guchhait P (2015) Type 2 diabetes: current understanding and future perspectives. IUBMB Life 67(7):506–513. https://doi.org/10.1002/iub.1396
- Kan C, Jayaweera K, Adikari A et al (2020) Genetic overlap between type 2 diabetes and depression in a Sri Lankan population twin sample. Psychosom Med 82(2):247–253. https://doi.org/10.1097/PSY.0000000000000771
- Smagula S, Stahl S, Santini T et al (2020) White matter integrity underlying depressive symptoms in dementia caregivers. Am J Geriatr Psychiatry 28(5):578–582. https://doi.org/10.1016/j.jagp.2019.11.010
- Victoria L, Alexopoulos G, Ilieva I et al (2019) White matter abnormalities predict residual negative self-referential thinking following treatment of late-life depression with escitalopram: a preliminary study. J Affect Disord 243:62–69. https://doi.org/10.1016/j.jad.2018.09.013
- Repple J, König A, de Lange S et al (2021) Association between genetic risk for type 2 diabetes and structural brain connectivity in major depressive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. https://doi.org/10.1016/j.bpsc.2021.02.010
- Flynn E, Tanigawa Y, Rodriguez F, Altman R, Sinnott-Armstrong N, Rivas M (2021) Sex-specific genetic effects across biomarkers. Eur J Hum Genet 29(1):154–163. https://doi.org/10.1038/s41431-020-00712-w
- Chan J, Wong C, Or P, Chen E, Chang W (2021) Risk of mortality and complications in patients with schizophrenia and diabetes mellitus: population-based cohort study. Br J Psychiatry 219(1):375–382. https://doi.org/10.1192/bjp.2020.248
- Yuan S, Larsson S (2020) An atlas on risk factors for type 2 diabetes: a wide-angled Mendelian randomisation study. Diabetologia 63(11):2359–2371. https://doi.org/10.1007/s00125-020-05253-x
- Nordsletten A, Larsson H, Crowley J, Almqvist C, Lichtenstein P, Mataix-Cols D (2016) Patterns of nonrandom mating within and across 11 major psychiatric disorders. JAMA Psychiatry 73(4):354–361. https://doi.org/10.1001/jamapsychiatry.2015.3192
- Martin AR, Gignoux CR, Walters RK et al (2017) Human demographic history impacts genetic risk prediction across diverse populations. Am J Hum Genet 100:635–649. https://doi.org/10.1016/j.ajhg.2017.03.004
- Duncan L, Shen H, Gelaye B et al (2019) Analysis of polygenic risk score usage and performance in diverse human populations. Nat Commun 10(1):3328
- Li H, Zhang C, Hui L et al (2021) Novel risk loci associated with genetic risk for bipolar disorder among Han Chinese individuals: a genome-wide association study and Meta-analysis. JAMA Psychiatry 78(3):320–330. https://doi.org/10.1001/jamapsychiatry.2020.3738
- Ikeda M, Takahashi A, Kamatani Y et al (2018) A genome-wide association study identifies two novel susceptibility loci and trans population polygenicity associated with bipolar disorder. Mol Psychiatry 23(3):639–647. https://doi.org/10.1038/mp.2016.259
- Martin A, Kanai M, Kamatani Y, Okada Y, Neale B, Daly M (2019) Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet 51(4):584–591. https://doi.org/10.1038/s41588-019-0379-x
Authors’ relationships and activities
CYC is an employee of Biogen. The remaining authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
This work was supported by the Taiwanese National Health Research Institutes (NHRI-EX109-10931PI and NHRI-EX110-10931PI).
Author information
Affiliations
- Department of Occupational Safety and Health, College of Public Health, China Medical University, Taichung, Taiwan
Mei-Hsin Su & Shi-Heng Wang
- Department of Public Health, College of Public Health, China Medical University, Taichung, Taiwan
Ying-Hsiu Shih, Pei-Chun Chen & Shi-Heng Wang
- Center for Neuropsychiatric Research, National Health Research Institutes, Miaoli, Taiwan
Yen-Feng Lin & Yu-Li Liu
- Biogen, Cambridge, MA, USA
Chia-Yen Chen
- Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, MA, USA
Chia-Yen Chen
- College of Public Health, National Taiwan University, Taipei, Taiwan
Po-Chang Hsiao & Po-Hsiu Kuo
- School of Medicine, College of Medicine, China Medical University, Taichung, Taiwan
Yi-Jiun Pan
- Department of Psychiatry, Taipei Veterans General Hospital, Taipei, Taiwan
Shih-Jen Tsai
- Department of Psychiatry, National Taiwan University Hospital, Taipei, Taiwan
Chi-Shin Wu
- Institute of Statistical Science, Academia Sinica, Taipei, Taiwan
Yen-Tsung Huang
Contributions
SHW conceptualised and designed the study. MHS and YHS drafted the manuscript and performed the data analysis. YFL, PCC, CYC, PCH, YJP, YLL, SJT, PHK, CSW and YTH interpreted the results and critically revised the draft. All authors reviewed and approved the final manuscript. SHW is responsible for the integrity of the work as a whole.
Corresponding author
Correspondence to Shi-Heng Wang.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.